Lithium button batteries, also known as coin cell batteries, operate based on the principles of electrochemistry. Here's a simplified explanation of how they work:
- Electrolyte: The battery consists of an electrolyte, which is a substance that allows the flow of charged particles, called ions, between the positive and negative electrodes.
- Positive electrode (Cathode): The positive electrode of a lithium button battery is typically made of a lithium compound, such as lithium cobalt oxide (LiCoO2) or lithium manganese dioxide (LiMnO2). This electrode receives electrons during discharge.
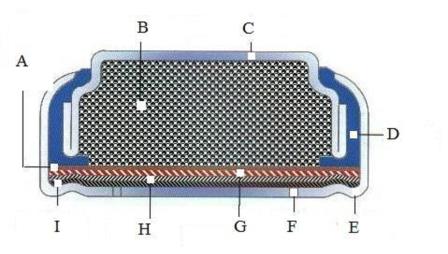
A:Separator, B: zinc powder anode and electrolyte, C: anode can, D: insulator gasket, E: cathode can, F: air hole, G: cathode catalyst and current collector, H:air distribution layer, I: Semi permeable membrane
- Negative electrode (Anode): The negative electrode, also known as the anode, is typically made of metallic lithium. When the battery is discharged, lithium ions leave the anode and move through the electrolyte to the cathode.
- Separator: A separator is placed between the positive and negative electrodes to prevent direct contact. It allows the flow of lithium ions while preventing the direct flow of electrons, maintaining the integrity of the electrochemical reactions.Ion movement: During discharge, lithium ions move from the anode through the electrolyte to the cathode. Simultaneously, electrons flow through an external circuit, creating an electric current that can power a device.
- Recharging: Some types of lithium button batteries are rechargeable. During the recharging process, an external power source, such as a charger, applies a higher voltage to the battery, causing the lithium ions to move back to the anode, effectively reversing the discharge process. This allows the battery to be used again.
Post time: Jul-07-2023
